
| AIR & PRODA | COLD CHAIN | COVERAGE RATES | COVID-19 | EDUCATION | INDIGENOUS |
| INFLUENZA | NEWS | NIP RESOURCES | NURSES | PHARMACISTS | RACF |
Following the conclusion of the CVTP training platform, two reference documents are avaialble to support providers :
From 1 October 2023, the COVID-19 Vaccination Training Program (CVTP) will be coming to an end. Read article.
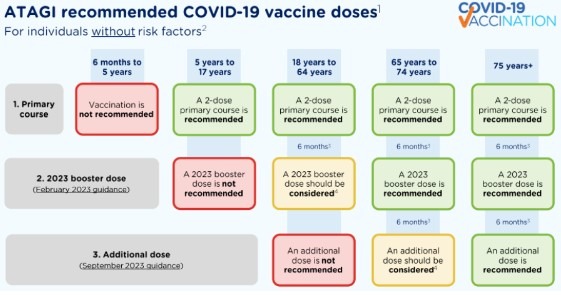
On 1 September 2023, ATAGI released recommendations about an additional 2023 dose of a COVID-19 vaccine. Read article.
On 30 May 2023, ATAGI advised that a bivalent COVID-19 vaccine is now preferred over original (ancestral) vaccines for primary vaccination in people aged 12 years or older. Read article.
On 8 February, ATAGI released recommendations about a 2023 booster dose for adults if their last COVID-19 vaccine dose was 6 months ago or longer, or their last confirmed infection was 6 months ago or longer. Read article.
All vaccines involved in a potential CCB, either within the clinical setting or during transit, must be reported to the Vaccine Operations Centre (VOC) as soon as possible.
Do not use or discard the vaccines until you have been advised to do so.
If you require assistance, the VOC can be contacted on 1800 318 208 during operating hours, 9.00am to 5.30pm Monday to Friday (AEST).
Practices can submit a request to access to more vaccine products within CVAS. Check current access via the ‘View Existing Vaccine Access’ button which lists a summary of all vaccine products the site is currently approved to order.
How to submit a request to update vaccine access:
ATAGI guidelines on the management of a range of possible vaccine administration errors, including when a replacement (repeat) dose is recommended.
ATAGI advice on use of sedation for COVID-19 vaccination provides an overview of the use of sedation as one of a range of measures to assist in the safe administration of COVID-19 vaccines, in patients with anxiety disorders or needle-phobia.
The NSW Multicultural Health Communication Service’s online Appointment Reminder Translation Tool allows you to translate appointment details into your client’s language.
Please refer to HealthPathways for the current definitions of low, medium and high risk. These definitions will be frequently updated and will differ between the two LHDs
COVID-19 vaccine brand that is not available in Australia
ATAGI advice People who have received either a first dose or a full course of a COVID-19 vaccine that is not TGA-approved or TGA-recognised should be offered two doses of an alternative TGA-approved vaccine brand available in Australia to be considered fully vaccinated. People should begin their full course of a TGA-approved or recognised COVID-19 vaccination at an interval of 4 to 12 weeks after their most recent COVID-19 vaccine dose.
ATAGI guidelines on the management of a range of possible vaccine administration errors, including when a replacement (repeat) dose is recommended.
Don’t forget to check – Poster
Advice relating to patient:
Advice relating to practice:
Clinical advice:
If an error has occurred during vaccine preparation or vaccine administration, resulting in an inadequate two-dose primary course, another dose may be recommended. The aim of replacement doses is to attain a level of immune response that is comparable to that expected following completion of a two-dose primary course of a COVID-19 vaccine according to the recommended dosage and schedule (ATAGI, 2021u).
| Vaccine Administration Error | ATAGI Recommendation |
|---|---|
Less than half of the vaccine dose volume (estimated) was administered |
Give a replacement dose a minimum of 1 week after the invalid dose, and a subsequent dose as indicated |
Incorrect diluent (such as sterile water for injection) used to dilute Pfizer (COMIRNATY) (For Age 5 to <12 Years) vaccine dose |
Give a replacement dose of Pfizer (COMIRNATY) (For Age 5 to <12 Years) a minimum of 1 week after the invalid dose, and a subsequent dose as indicated |
Only the diluent of Pfizer (COMIRNATY) (For Age 5 to <12 Years) was administered (i.e. no Pfizer (COMIRNATY) vaccine ingredient) |
Give a replacement dose of Pfizer (COMIRNATY) (For Age 5 to <12 Years) as soon as feasible, and a subsequent dose if indicated |
If the 2nd dose of Pfizer (COMIRNATY) (Age 5 to <12 Years) is administered less than 14 days after the first dose, it is considered an invalid dose. |
An additional dose of Pfizer (COMIRNATY) (For Age 5 to <12 Years) should be administered as a replacement dose. The interval between the invalid 2nd dose and the replacement dose is flexible, recommended at 4 to 12 weeks after the invalid 2nd dose (ATAGI, 2021u). |
Translated resources
Supporting communication for the COVID-19 vaccination program – This glossary was developed to help community organisations, translators and interpreters, bilingual workers, and community leaders to better understand and communicate about vaccine development and implementation.
Information for teens/kids
Guidance to help people make informed decisions
Information for Aboriginal and Torres Strait Islander peoples
The CESPHN Vax at Home Service is ONLY for those people who are housebound and not able to leave their house to receive a COVID-19 vaccination or booster (their carers will also be able to be vaccinated).
Patient self-referrals will NOT be accepted. Patients should speak with their GP for referral to this program if appropriate. Referrals will be accepted from general practitioners and from Local Health Districts. More information.
The Vax at Home Service will accept referrals for people 16 years and over. For children 12-16 years with special circumstances and may require further support, referrals will be reviewed on a case by case basis.
Referrals
Referrals MUST be sent through this Vax at Home Service Online Form – for people who are housebound and reside in the Sydney Local Health District or South Eastern Sydney Local Health District regions.
For further information (no referrals) on the program, please email vaxathomeservice@cesphn.com.au
See CESPHN Immunisation – RACF webpage
See CESPHN Immunisation – Indigenous webpage
Provider resources
Access to COVID-19 vaccines
Submitting an Expression of Interest for Commonwealth in-reach (VAPP)
Disability service providers may submit an expression of interest to the Department of Health for Commonwealth in-reach support, where this is still the most appropriate vaccination service. Disability Service Providers should submit the form; or, for individuals in need of in-home support, the PHN can submit the form on their behalf.
Once received, a VAPP provider will be allocated to support the disability service provider or individual with their vaccination needs. Where to go for more information More information on the Winter dose, vaccination options, and the in-reach program for people with disability are available on the Department of Health’s website. Enquiries can also be sent to DisabilityCovidVaccineDelivery@health.gov.au
Aged 60 years and over
Aged under 60 years
Visit website to find out how to book your vaccination appointment.
Support Contacts
Need support? See COVID-19 Q&A April 2020
NSWISS clinical Support: NSW Immunisation Specialist Service
VOC: Vaccine Operations Centre
VCF connect
CVAS: COVID-19 Vaccine Ordering System
Department of Health COVID-19 support:
Translating service
GPRC support
CESPHN COVID-19 support:
Posters:
• Pfizer Bivalent Comparison poster
• AIR Codes Table
• Don’t Forget to Check
• COVID-19 Vaccine Storage poster
Other:
• COVID-19 Vaccine Transfer Policy
• Service Finder and Online Bookings
• MBS Payments
• Australian Immunisation Register (AIR) for COVID-19 Vaccine
• Pfizer 6 months to 4 years (Maroon Cap)
• Pfizer 5-11 years (Orange Cap)
• Pfizer Bivalent BA.1 18 years+ (grey cap)
• Pfizer Bivalent BA.4-5 12 years+ (grey cap)
• Moderna Bivalent BA. 4-5 PFS
• Novavax
All immunisation providers must complete the additional age specific COVID-19 vaccine for children training modules.
Patients resources

Community resources
Provider resources
Provider training
Provider information
Patient information
Provider training
Provider information
Patient information
Patient information
Pericarditis and Myocarditis
Provider information (adult)
Provider resources (children)
Patient information (children)
Patient information (adult)
Patient information
Thrombosis with thrombocytopenia syndrome (TTS)
ATAGI clinical guidance for COVID-19 vaccine providers
On 1 September 2023, ATAGI released recommendations about an additional 2023 dose of a COVID-19 vaccine. More information.
On 30 May 2023, ATAGI advised that a bivalent COVID-19 vaccine is now preferred over original (ancestral) vaccines for primary vaccination in people aged 12 years or older. Read full article.
Novavax vaccines with Lot No. 4302MF031 has been recalled due to a potential issue with the batch. There are no patient safety concerns associated with the recalled vaccine, however it is still recommended not to use the affected batch.
The following COVID-19 vaccines are no longer available in Australia:
Vaccine wastage refers to the loss of vaccine doses due to cold chain breaches, expired vials or other damage.
The COVID-19 Vaccination Training gives details on how to dispose of vaccines:
Remember to notify wastage to the Vaccine Operations Centre (VOC) through the COVID-19 Vaccine Administrative System (CVAS):
Pfizer 12+ shelf-life extension – some batches of Pfizer with manufacturer expiry 28 Feb 2022 have had their shelf life extended. Check information sheet before discarding vials.
COVID-19 vaccines given overseas that have been recognised by the Therapeutic Goods Administration (TGA) can now be recorded on the Australian Immunisation Register (AIR).
It is important that country of immunisation and batch number are recorded. A list of all vaccines currently able to be reported to AIR is available on the AIR vaccine code page which is updated regularly.
The AIR042 report identifies patients:
Further information:
The Practice Incentives Program (PIP) COVID-19 in-reach vaccination payment is a time-limited payment to support general practices that undertake in-reach COVID-19 vaccination services for residential aged care and disability support workers in their workplace.
This payment is only available for Medicare Benefits Schedule (MBS) COVID-19 vaccine suitability assessment services (for primary vaccine course and subsequent booster vaccine doses) that are administered via an in-reach COVID-19 vaccination clinic between 29 April 2021 to 30 June 2022. See payment guidelines.
Tool to address vaccine concerns: CoRiCal
Covid Risk Calculator (CoRiCal)
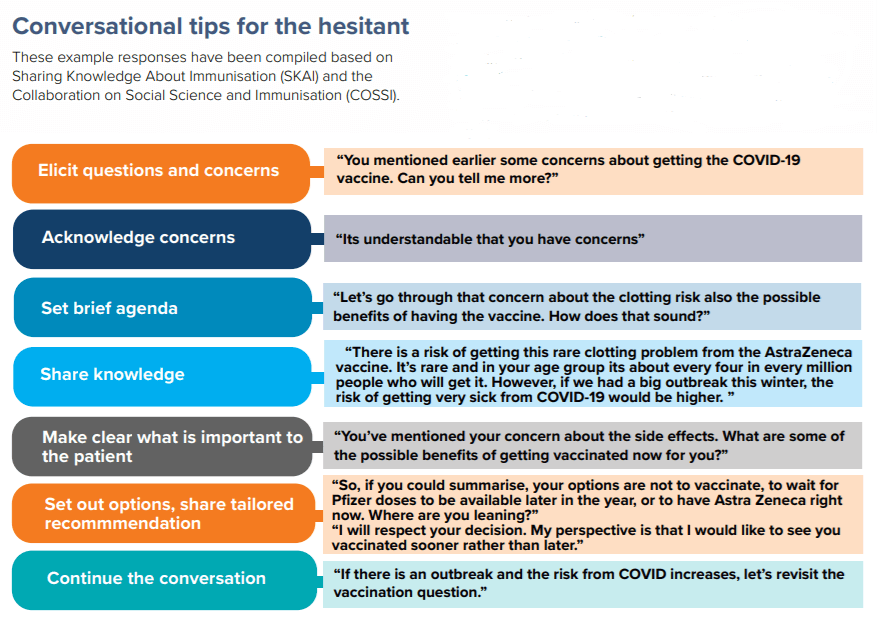
Understanding and addressing vaccine hesitancy
Resources to address vaccine hesitancy
Communication tools to address vaccine hesitancy
NSW Health is currently responding to a number of COVID-19 outbreaks all across NSW. Healthcare workers are at high risk of exposure to COVID-19.
The following guides should be adapted to suit your practices procedures and workflows:
Subscribe to Sydney Health Weekly to receive immunisation updates
SUBSCRIBE NOW to our weekly Immunisation email updates via Sydney Health Weekly
